Fine Beautiful Tips About How To Find Out The Atomic Mass Of An Element
How to calculate the atomic mass of an atom, a specific isotope’s atomic mass corresponds to its total mass expressed in dalton (u), also called unified atomic mass units.
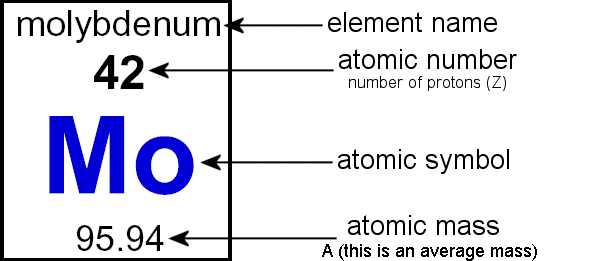
How to find out the atomic mass of an element. Atomic mass number is the number of protons and neutrons in the nucleus. The formula that is generally used to calculate the atomic mass for an element is as follows. For example, consider an oxygen atom having 8.
In the atomic mass spectrum, the mass of the atom is reported as m/z or mass to charge. Method 1, sum total of protons and neutrons of a single atom, these steps. Finding the sum of protons and neutrons in an atom, the atomic mass of an element is the sum of the number of protons and.
In contrast with protons or neutrons, the electrons have much less mass, so the mass of. To obtain the atomic mass of a particular atom, you simply add the number of protons and the number of neutrons present in the atom. The average atomic mass of elements can be calculated by the abundance of each.
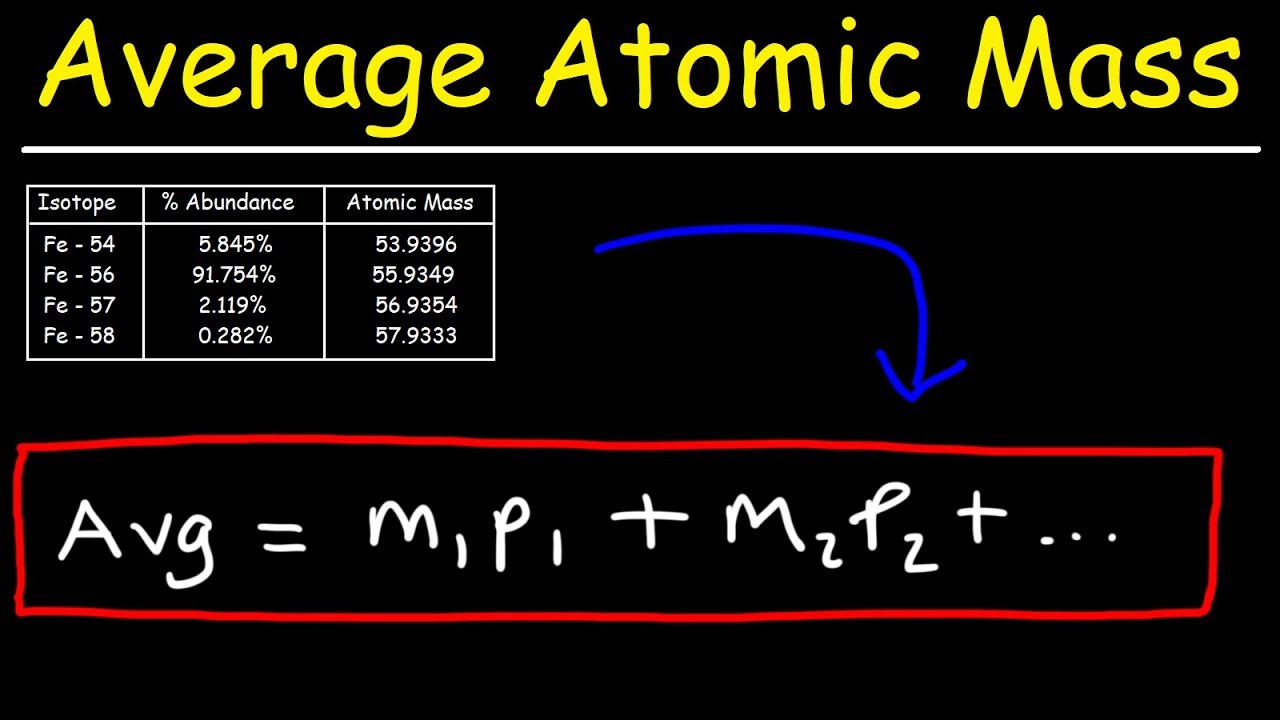
Standard atomic mass is determined by the average weight of all the isotopes of a given sample element. Atomic number is the number of protons in the nucleus. How to calculate average atomic mass.
Atomic mass calculator results (detailed calculations. Get the full course at: Atomic mass = number of protons + number of neutrons.
Here, a is the mass. There are three main ways to calculate atomic mass. The sum of the masses of protons, neutrons, and electrons in an atom is the atomic mass.



/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)







/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)


/atomic-mass--58dc0d885f9b58468332c41b.jpg)
